Hydrochloric acid
Hydrochloric acid (HCl) is a clear colorless liquid that is highly corrosive, and considered a strong mineral acid. The main applications of HCl are for pickling steel, acid treatment for oil wells, chemical cleaning, and chemical processing for large scale production of vinyl chloride. It is produced in several concentrations with the max being 38%. It is possible to produce concentrations above 38%, but at higher concentrations the evaporation rate increases dramatically and special storage and handling are required.
When trying to understand the degradation of materials in an HCl environment, it becomes very complex because small amounts of metal chlorides and impurities can vastly affect the corrosive nature of the solution. Oxidizing agents greatly increase the corrosion rate of metals and alloys, and also organic contaminates detrimentally affect plastics and elastomers. The table below is a general guideline of some alloys to consider based on their performance in an HCl environment. As previously mentioned, however, impurities in HCl can drastically alter these guidelines.
| Not Suggested | Good | Better | Best |
|---|---|---|---|
| 304, 316, 2205, 20 | 200, 400, 625, ZERON® 100, AL-6XN® | C-276, C22 | Zirconium, Tantalum, B-2 |
Metals such as aluminum, cast iron, steel, copper, and titanium will suffer rapid attack from HCl at all concentrations and temperatures. Most stainless steel grades will be subject to attack, because their chromium content is not sufficient in forming a protective passive layer. Without the passive layer the stainless steel will then begin to corrode actively, which leads to rapid corrosion rates, pitting, and stress corrosion cracking. However, some 6% Mo and super duplex grades have been used to handle dilute solutions of HCl at ambient temperatures.
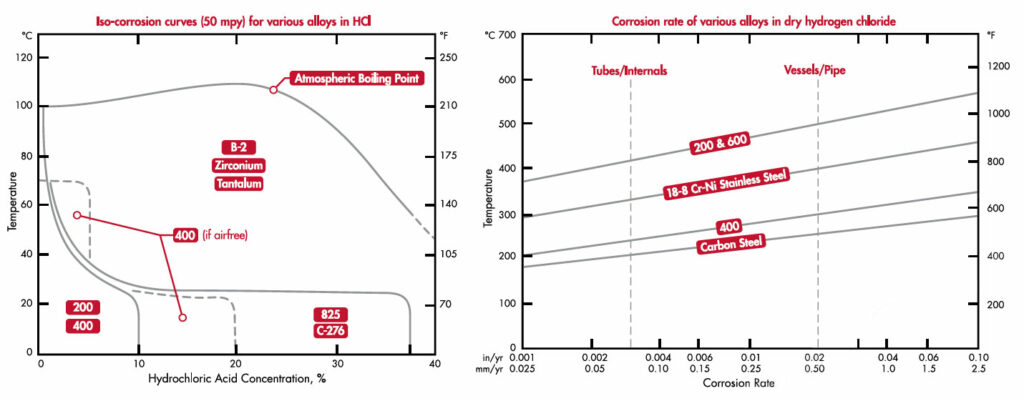
The figure below left is a corrosion curve of nickel and reactive alloys with respect to temperature and concentration in HCl. In general, nickel alloys have a better resistance than stainless steel, but still not completely corrosion resistant for most concentrations. Only C-276 is able handle most concentrations of HCl at room temperature. Nickel molybdenum alloys, such as B-2 and B-3® have good resistance at all concentration and temperatures. However, oxidizing contaminates have the ability to take alloys B-2 and B-3® from very resistant to highly susceptible to attack, and these oxidizing contaminates are difficult to exclude from practice. Reactive metals offer the best resistance to HCl. Both zirconium and tantalum will offer the most resistance to HCl at all concentrations and temperatures. Applications for piping, valves, pumps and gaskets would generally use reactive metals or nickel alloys.
Nonmetallic materials are generally preferred for handling and storing HCl. Plastics, such as polyethylene, polypropylene, polyvinyl chloride are resistant, but are limited to ambient temperatures. Fiberglass-reinforced plastic (FRP) is also resistant and has been generally used for the storage and transportation. Graphite and rubber have also been used for similar applications as other nonmetallic materials, due to their similar corrosion resistance as plastics and FRP.
Glass and other ceramic materials are able to withstand HCl up to its boiling point. The major pitfall of glass is when it is used to line metal, where it can potentially fail from external corrosion from spillage or vapors that would corrode the outside metal and molecular hydrogen would form from the corrosion process and permeate into the metal. The hydrogen would migrate to the metal-glass interface, and the resulting build up of hydrogen would create a highly pressurized area of gas that would pop off the glass lining.
Dry hydrogen chloride is typically non-corrosive. Generally the main concern is if the temperature was to drop substantially and the dry hydrogen chloride condenses and HCl forms. The figure above right is a graph that represents the material selection for various alloys in a dry hydrogen chloride environment. Carbon steel can be used up to temperatures around 480°F. Above that temperature, chromium based alloys with good oxidation resistance are more commonly used. At temperatures above 1000°F alloys such as 310, RA330®, 600, and RA 602 CA® are also used depending on the temperature and concentration of the environment.