MICRobial corrosion
Microbiologically Influenced Corrosion (MIC)
The observation that bacteria had an impact on corrosion was first made more than a century ago, and was confirmed on many occasions over the last thirty years. It is important to note that the bacteria do not consume the metal, but their metabolic by-products can change the local environment sufficiently to result in corrosion. Hence, the name microbiologically influenced corrosion, or MIC as it is commonly known. In 1983, MIC was estimated to be costing the world economy 30 to 40 billion dollars annually.
All natural, surface-derived waters (both seawater and fresh water) contain bacteria, although the types and concentrations vary with the location and temperature. Borehole waters in the UK are usually well aerated and low in bacteria, although they often contain sulfate reducing bacteria, which will become active if the dissolved oxygen content becomes very low. This will then result in significant quantities of hydrogen sulfide in the water, produced by the bacteria.
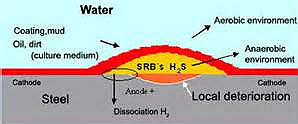
Once the first bacteria colonize a surface, they produce extracellular material that typically consists of polysaccharide polymers. As the biofilm grows and thickens, the aerobic bacteria consume the oxygen near the metal/biofilm interface and anaerobic bacteria become active. In the outer layer of the biofilm, oxygen can still penetrate and aerobic bacteria are active. The secondary colonization of the biofilm can promote or inhibit the activity of the primary bacteria depending on the type.
Corrosion only occurs once an effective micro-colony is established and an electrochemical potential difference is established between different areas on the metal surface. The growth and development of biofilms are affected by the temperature, pH, water velocity and surface roughness. Most bacteria thrive best within a limited range of temperature and pH, although these can vary significantly from species to species. Bacteria do not adhere well to metal surfaces if the water flow is high or the surface is very smooth.
Types of Bacteria
Microbes in seawater can influence corrosion in one of five ways:
- Production of aggressive metabolic products, such as sulfuric acid, or chelating agents.
- Cathodic depolarization associated with anaerobic growth.
- Changes in oxygen potential, salt concentration, pH etc, which establish local electrochemical cells.
- Removal of corrosion inhibitors or protective coatings.
- The biomass itself stimulates attack, for example by creating an occluded cell. Where the water is not chlorinated, or otherwise treated to inhibit biological activity, biological colonization occurs rapidly.
Colonization starts within hours and becomes well established in periods from a few days to a few weeks, depending on local conditions.
There are cathodically depolarizing biofilms that form on stainless steels in natural waters, and these change the local redox potential and, hence, the corrosion behavior. There are other bacteria that thrive under oxidizing conditions i.e. in aerated water. One type is the iron oxidizing bacterium. This works by creating a differential aeration cell and it is usually a problem with cast iron and carbon steel. Stainless steels are more resistant to this type of attack, but problems have been reported with stainless steels in the presence of manganese oxidizing bacteria. These oxidize manganese in solution in the water to manganese dioxide, which deposits on the metal surface. Manganese dioxide is very efficient at reducing dissolved oxygen, the most common cathodic reaction in aerated waters.
Stainless steels have a thin protective film (primarily chromium oxide), which is continually breaking down and re-forming in service. The presence of an efficient cathode, like manganese dioxide, can greatly increase the chances of localized corrosion initiating and propagating following a film breakdown event.
Note that the addition of a strong oxidizer, such as chlorine, to water to control MIC, may result in the exact same problem, as chlorine readily oxidizes manganese in solution to manganese dioxide. This is more likely to happen in waters with high dissolved manganese concentrations. Another type of bacterium that thrives under aerated conditions is the sulfur-oxidizing species (SOB), which creates sulfuric acid as a by-product. This will cause severe attack of carbon steel and may cause corrosion of lower alloy stainless steels. Hence, alloys that are resistant to sulfuric acid at all concentrations should be resistant to this type of attack.
When the water is stagnant, the aerobic bacteria will consume the available oxygen and then the anaerobic bacteria will become active. The most well-known are the sulfate reducing bacteria (SRB), which produce H2S as a by-product. The effect of H2S is to reduce the potential for pitting/crevice corrosion of stainless steels in the presence chloride ions. The susceptibility to attack related to SRB increases as the chloride concentration and temperature increase.
Effects of Bacteria on Corrosion
It is well documented that when a stainless steel is immersed in a natural water, the potential increases over a period of a few days to weeks, stabilizing in the range of +250 to +400mV SCE. This has been found for both seawater and fresh waters. The ennoblement is due to the formation of a biofilm that cathodically depolarizes the reduction of dissolved oxygen. The exact mechanism has not been determined, but it is currently believed that hydrogen peroxide is produced as part of the metabolic process. This is an oxidizer and additions of peroxide to chloride solutions result in the same kind of potential increases for stainless steels as those seen in natural waters. In natural seawater (after the biofilm forms), even a small change in potential results in high corrosion currents, while without the biofilm, a similar potential change results in much smaller corrosion currents. If a biocide, such as chlorine, is added, the biofilm does not form, but the higher redox potential results in a higher open circuit potential. The cathodic reaction is now the reduction of hypochlorite to chloride, which is not as efficient as the reduction of dissolved oxygen with a biofilm, resulting in lower currents than when the biofilm is present.
These electropositive potentials can mean that an alloy is taken past its pitting or crevice potential. The potential of such materials then decreases and this is often used as an indicator that crevice corrosion has initiated. With the sulfur oxidizing bacteria, it is the sulfuric acid, produced as a metabolic byproduct that causes the corrosion. Resistance to this type of attack can be assessed by examining an alloy’s resistance to sulfuric acid over a wide range of concentrations, at the temperature of interest.
With SRB, the H2S produces local reducing conditions and negative potentials and has been observed in wastewater environments. In the early stages a biofilm forms that produces electropositive potentials, similar to those seen in seawater. However, over time the potential slowly decreases to very negative values. This was attributed to the formation of a thicker biofilm, such that SRB became active beneath it. In some instances, even with this electronegative potential, no localized corrosion developed.
However, corrosion did initiate in waste water plants that added oxidizing chemicals, such as potassium permanganate, as part of the waste treatment process. This can change the local redox potential such that corrosion can occur because sulfide can lower the threshold potential for localized corrosion.
Environmental Variables
There are a number of variables that can affect the onset of MIC. It should be noted that just because bacteria are present in water, it does not mean that they are active. They must have a suitable supply of nutrients and a hospitable environment. Temperature and pH must remain within ranges that can sustain bacterial growth. This means that it is difficult to ascribe a particular failure categorically to MIC. It also means that reproducing MIC is difficult because small changes in the conditions may not favor proliferation of a particular species.
High flow velocities will deter the formation of colonies which are necessary for the initiation of MIC. Work has been performed to show that flow rates up to 1.6 m/s (5.2 fps) developed similar colonization, even with intermittent stoppages.
Another significant factor that is well documented is surface finish. Bacteria find it easier to attach to rough rather than smooth surfaces. It is not that attachment will not occur on smooth surfaces, but it is more difficult and usually takes longer. Exposure tests on a number of stainless steels in fresh water found that the rougher surfaces were more easily colonized than polished surfaces. It has also been found that welds and the HAZ are favored colonization sites. Corrosion can also initiate more easily at the HAZ because the heat tint leaves a thin, chromium depleted layer on the metal surface beneath it. The lower chromium content decreases the PREN and increases the risk of localized corrosion initiating there. Removing heat tint by pickling or mechanically grinding can be beneficial in such circumstances.
Service Experience
There are many published experiences of MIC failures. Most of these relate to 300 series austenitic stainless steels. Some of the references posted at the end of this discussion report these failures in detail.
Since MIC is essentially a mechanism to develop more severe corrosion conditions (decreased pitting potentials, corrosion cells, anaerobic conditions, etc.) than might otherwise be expected to exist, it follows that more corrosion resistant alloys can be used to combat the corrosive effects of MIC.
As noted earlier, increased resistance to sulfuric acid, H2S and chloride pitting or crevice corrosion may be beneficial in combating the corrosive environments created by bacterial colonies. 316, 304 and lesser alloyed materials are known to be susceptible. MIC has also been identified in 321 and 317L. More highly alloyed materials such as 2205 and 904L have exhibited mixed performance. MIC of 2205 was noted in a wastewater treatment system where chloride levels were over 1000 mg/l.
In multiple cases, failures of 304 or 316 replaced with AL-6XN© Alloy, or a superduplex, like ZERON 100© eliminated corrosion problems from MIC. AL-6XN Alloy has been used extensively to replace service water piping systems which are particularly susceptible to MIC corrosion. These alloys offer significantly higher resistance to chloride pitting and crevice corrosion than the 300 series stainless grades. They also offer good resistance to sulfuric acid in a broad concentration range at temperatures above where most bacteria will survive. Even more highly alloyed materials, such as 625 and the C-type alloys are resistant to MIC although the need to go to such materials has not been demonstrated.